
Active vocabulary
Date: 2015-10-07; view: 670.
UNIT 2. Chemical Properties of Petroleum
C. Discussion
Topics:
1. Reservoir development: stages of petroleum formation.
2. Types of oil: their physical and chemical properties.
3. Main types of reservoir rocks and their textual properties.
| 1. | constituent | составная часть |
| 2. | carbon | углерод |
| 3. | hydrogen | водород |
| 4. | sulfur | сера |
| 5. | nitrogen | азот |
| 6. | sodium chloride | хлористый натрий |
| 7. | residue | остаток; осадок; вещество, оставшееся после сгорания или выпаривания |
| 8. | density | плотность, удельный вес |
| 9. | integer | целое число |
| 10. | benzene | бензол |
| 11. | gravity | плотность |
| 12. | spore | спора |
| 13. | resin | смола, канифоль, камедь |
| 14. | remnant | остаток |
| 15. | excess | избыток, излишек |
| 16. | abundant | богатый, обильный, изобильный |
| 17. | to range from … to … | колебаться от … до … |
| 18. | to predominate | преобладать |
| 19. | to generate | порождать, производить |
| 20. | to comprise | содержать, вмещать |
| 21. | to obtain | получать |
| 22. | to incorporate | объединять(ся), соединять(ся) |
| 23. | to release | выпускать |
| 24. | a saturated straight-chain series | соединение с нормальной неразветвленной цепью |
| 25. | asphalt-base crudes | нефть асфальтового основания |
| 26. | viscous asphalts | полужидкий битум |
| 27. | lower density paraffins | парафины с более низкой плотностью |
| 28. | a saturated closed-ring series | насыщенный циклический ряд |
| 29. | plastic and solid paraffin waxes | пластические и твердые парафины |
| 30. | liquid refinery products | жидкие нефтепродукты |
| 31. | medium and heavy fractions | средние и тяжелые фракции |
| 32. | low and medium molecular ranges | низкие и средние молекулярные ряды |
| 33. | combustion of oil | горение нефти |
| 34. | decay-resistant organic remains | неразложившиеся органические остатки |
| 35. | organic combinations | органические соединения |
| 36. | siliceous skeletal fragments | фрагменты скелетов, содержащие кремний |
Although oil consists basically of compounds of only two elements, carbon and hydrogen, these elements form a large variety of complex molecular structures. Regardless of physical or chemical variations, however, almost all crude oil ranges from 82 to 87 percent carbon by weight and 12 to 15 percent hydrogen. The more viscous asphalts generally vary from 80 to 85 percent carbon and from 8 to 11 percent hydrogen.
Crude oil can be grouped into three basic chemical series: paraffins, naphthenes, and aromatics. Most crude oils are mixtures of these three series in various and seemingly endless proportions. No two crude oils from different sources are completely identical.
The paraffin series of hydrocarbons, also called the methane (CH 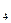 ) series, comprises the most common hydro-carbons in crude oil. It is a saturated straight-chain series that has the general formula CnH2n+2, in which С is carbon, H is hydrogen, and n is an integer. The paraffins that are liquid at normal temperatures but boil between 40° and 200° С (approximately between 100° and 400° F) are the major constituents of gasoline. The residues obtained by refining lower density paraffins are both plastic and solid paraffin waxes.
) series, comprises the most common hydro-carbons in crude oil. It is a saturated straight-chain series that has the general formula CnH2n+2, in which С is carbon, H is hydrogen, and n is an integer. The paraffins that are liquid at normal temperatures but boil between 40° and 200° С (approximately between 100° and 400° F) are the major constituents of gasoline. The residues obtained by refining lower density paraffins are both plastic and solid paraffin waxes.
The naphthene series has the general formula CnH2n and is a saturated closed-ring series. This series is an important part of all li.,lkoi98quid refinery products, but it also forms most of the complex residues from the higher boiling point ranges. For this reason, the series is generally heavier. The residue of the refinery process is asphalt, and the crude oils in which this series predominates are called asphalt-base crudes.
The aromatic series has the general formula CnH2n - 6 and is an unsaturated closed-ring series. Its most common member, benzene (C6H6), is present in all crude oils, but the aromatics as a series generally constitute only a small percentage of most crudes.
In addition to the practically infinite mixtures of hydrocarbon compounds that form crude oil, sulfur, nitrogen, and oxygen are usually present in small but often important quantities. Sulfur is the third most abundant atomic constituent of crude oils. It is present in the medium and heavy fractions of crude oils. In the low and medium molecular ranges, sulfur is associated only with carbon and hydrogen, while in the heavier fractions it is frequently incorporated in the large polycyclic molecules that also contain nitrogen and oxygen. The total sulfur in crude oil varies from below 0.05 percent (by weight), as с in some Pennsylvania oils, to about 2 percent for average Middle Eastern crudes and up to 5 percent or more in heavy Mexican or Mississippi oils. Generally, the higher the specific gravity of the crude oil, the greater is its sulfur content. The excess sulfur is removed from crude oil during refining, because sulfur oxides released into the atmosphere during the combustion of oil would constitute a major pollutant.
The oxygen content of crude oil is usually less than 2 percent by weight and is present as part of the heavier hydrocarbon compounds in most cases. For this reason, the heavier oils contain the most oxygen. Nitrogen is present in almost all crude oils, usually in quantities of less than 0.1 percent by weight. Sodium chloride also occurs in most crudes and is usually removed like sulfur.
Many metallic elements are found in crude oils, including most of those that occur in seawater. This is probably due to the close association between seawater and the organic forms from which oil is generated. Among the most common metallic elements in oil are vanadium and nickel, which apparently occur in organic combinations as they do in living plants and animals. Crude oil also may contain a small amount of decay-resistant organic remains, such as siliceous skeletal fragments, wood, spores, resins, coal and lignite, and various other remnants of former life.
| <== previous lecture | | | next lecture ==> |
| B. Vocabulary practice | | | B. Vocabulary Practice |